Description
Pen-like non-contact tonometer for IOP measuring!
Diaton tonometer is a handheld device that measures IOP through the eyelid & completely satisfies present-day requirements of diagnostic equipment.
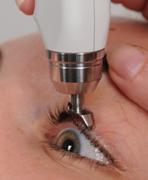
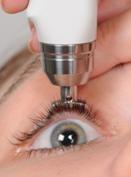
Spheres of diaton tonometer application
- ophthalmology (including children's)
- general medical practice
- optometry
- neurology
The Tonometer can be used in medical institutions including mass examinations only.
Method for measuring the intraocular pressure through the eyelid and the device realizing this method are protected by the Patent of Russia № 2123798, United States Patent № US 6,394,954 В1 and Patent of Japan №3593314.
Nowadays transpalpebral scleral tonometry has no other alternative in the world. It is the most favourable method to carry out preclinical medical research of the population and , moreover,it works in such complicated clinical cases when it is impossible to use classical tonometry methods. Diaton is a helpful tool for ophthalmologists faced with pediatric patients or who have corneal abnormalities such as corneal edema, erosions or keratoprosthesis.
Approved by the FDA in 2006, the instrument has been the subject of numerous clinical trials, where it has been found to be comparable with the gold standard, the Goldmann tonometer, and with many other devices.
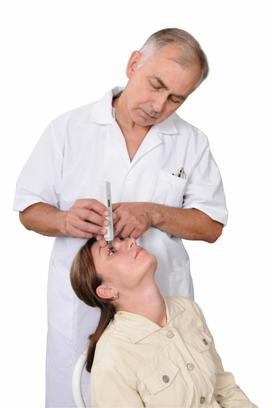 Innovative approach to IOP measurement opens up wide medical opportunities and indisputable benefits both for doctors & patients:
Innovative approach to IOP measurement opens up wide medical opportunities and indisputable benefits both for doctors & patients:
for doctors:
- Accurate results
- Wide range of medical opportunities
- Quick & efficient procedure of measuring (IOP measurement takes several seconds)
- Сreates an image of a modern doctor working in an up-to-date equipped medical center
- Wide medical opportunities
- Low dispensable materials' expenses
for patients:
- No contact to the cornea
- No risk of infection
- No anesthesia drops
- No pain
- No need to take out contacts
Transpalpebral "diaton" tonometer is effective and irreplaceable in complicated medical cases:
- Can measure IOP even in the presence of viral infections, allergic reactions, dry eye syndrome, contraindications for corneal tonometry
- Can serve as non-invasive day monitoring tool while selecting the adequate hypotensive medical treatment
- Can measure IOP on patients after corneal surgeries
- Сan measure IOP with contact lenses on
- Can measure IOP on immobilized patients
- Screening tool for elevated IOP by PCPs
| Features | diaton | ShiotzTonometer | Air-jet | Tonopen | Icare |
|---|---|---|---|---|---|
| No contact with the cornea | + | ||||
| Portability | + | + | + | + | |
| Displays independence from cornea's crookedness. |
+ |
||||
| Digital IOP indication | + | + | + | + | |
| Measurement in sitting position | + | + | + | + | |
| Measurement in reclining position | + | + | + | ||
| Short-time measurement | + | + | + | ||
| Sterilization is not required | + | + | + | ||
| Anesthesia is not required | + | + | + | ||
| Dispensable materials are not required | + | + | |||
| No need to take out the contact lens | + | - | - | - | - |
Comparative tests of diaton tonometer and Goldman tonometer give the evidence of high reliability that diaton tonometer guarantees.
SPECIFICATIONS
| Measurement range, mm Hg | 5-60 |
| Measurement error |
Limit of the admissible measurement error in the range, not more: from 5 to 20 mm Hg - ±2 mm Hg; from 20 to 60 mm Hg - ±10% |
| The time of a single measurement, s, not more | 3 |
| Supply voltage, V | 3 |
| Number of measurements using one battery set, not less | 1500 |
| Service life, not less | 5 |
| Weight, g | 89 |
| Dimensions, mm, not more | 174 х 26 х 20 |
Diaton tonometer is CE and FDA approved:
- CE
- Quality Management System ISO 13485 : 2003 & EN ISO 13485 : 2012
- Quality Management System - ISO 9001:2015
- FDA confirmation
User Manual:
|
|